Electrochemical Series
Electrochemical series, also known as activity series, is a list of metals listed in the order of reducing reactivity or reducing oxidation efficiency. Metals at the top of the series, alkali metals, and alkaline earth metals are more reactive or oxidized more readily than metals found at the bottom of the series.
This means that they can respond more easily to the formation of compounds. These metals at the top of the activity range are called active metals.
The metals at the bottom of the series, such as transition metals, are very stable and the compounds are less easily formed. These metals, such as copper and gold, are used to make coins and jewelery, and are known as noble metals because of their low reactivity. Elements in the electrochemical series are arranged according to their standard electrode potentials.
Electrode potential is the probability of a cell acting as an electrode cathode and acting as a standard hydrogen electrode (SHE) anode. Reduction always takes place at the cathode, and oxidation occurs at the anode.


Look at the series. Is it possible to predict whether a certain metal will be oxidized by an acid? This question is of practical importance as well as of chemical interest. For example, it’s unwise to store a solution of magnesium nitrate in an iron container because the solution will dissolve in the container.
Electrochemical series can also be called as e.m.f series.
Properties of the Electrochemical Series
Substances that are stronger than hydrogen are placed on hydrogen in series and have negative values of standard reduction energy(potential).
All substances that have positive values of reduction potential and are placed under hydrogen in the material are weakening agents than hydrogen.
Substances that are stronger oxidizing factors than the H + ion are placed under hydrogen in this series.
Metals on the top of hydrogen (which have high negative values of standard reduction potentials) have a tendency to lose electrons instantly. These are active metals.
The activity of metals decreases from top to bottom.
But Non-metallic metals have high positive values of standard reduction energy
There is a tendency to readily accept electrons. These are active metals.
But Non-metallic activity increases from top to bottom.
Let me discuss few examples related to it.
- Copper is above silver in the series. Thus, copper metal is oxidized by silver ions.

- The oxidation of copper metal to copper ions is accompanied by the reduction of silver ions to silver metal. For similar reasons, Copper(II) nitrate produces a blue color in the solution that occurs in the following reaction.

- Highly reactive metals such as sodium react with cold water to produce hydrogen and metal hydroxide:

Metals in the middle of the reactivity chain, such as iron, react with acids such as sulfuric acid (but not water at normal temperatures) and give metal salts such as

There is some ambiguity at the boundary lines between groups.
Magnesium, aluminum and zinc can react with water, but the reaction is usually very slow, unless the metal samples are specially made to remove the surface layer of oxide
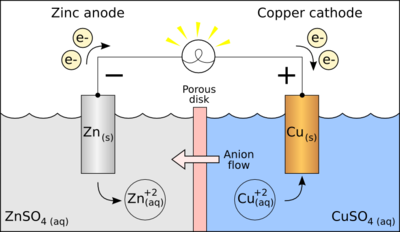
zinc deposited in the solution of copper sulphate quickly changes color with the deposition of metallic copper and the conversion of zinc into zinc (II) sulfate:


Generally, a metal reactivity displaces any metals that are low in the series: excess metal reduces the ions of the lower metal.
It is used in the thermite reaction and in the Kroll process for making titanium (similar to Al in the Ti reactivity series) for the manufacture of low-grade metallic iron.
For example, aluminum reduces iron (III) oxide to iron, in the process aluminum oxide:

Similarly, magnesium is used to extract titanium from titanium tetrachloride, a process in which magnesium chloride is formed.