Understanding Electrochemical Cells in chemistry
Electrochemical cells
Electrochemical cells are nothing but the devices used to generate an electric potential between electrodes.
In this tutorial, we’ll learn how to understand Electrochemical cells and how can we use them.
Every day, we start waking up with a mobile phone alarming and check our emails on our laptop which makes our lives easier and more enjoyable. But from where do these gadgets get the power.
From where we get this Power?
Electrochemical cells use the chemical reactions to generate electricity, or use electrical energy to cause chemical reactions.
Let’s move on to two main types of electrochemical cells – electrochemical and galvanic.

There are two types of Electrochemical cells:
- Galvanic cells ( or Voltaic Cells )
- Electrolytic cells
Electrochemical cells that generate electricity are called voltaic cells or galvanic cells, and those that produce chemical reactions are called electrolytic cells.
A common example of a galvanic cell is a 1.5-volt cell intended for consumer use. The battery consists of one or more cells, which are connected in parallel, series, or series-parallel designs.
Electrolytic cells:
Electrolytic cells that cause chemical reactions when electrical energy is applied. They consist of two electrodes, dipped in conducting fluid, a usually aqueous solution or molten salt. The power supply connects to the electrodes and provides the energy to cause a chemical reaction.

This process is called electrolysis. “lysis” is the word which has derived from the Greek that means “break up”.
Some of the examples of electrolysis are given below:
1. Decomposition of water into hydrogen and oxygen
2. Decomposition of bauxite into aluminum and other chemicals.
3. Electroplating (e.g. of copper, silver, nickel, or chromium) is done using an electrolytic cell.
Electrolysis is the main technique that uses the direct electric current (DC).
What is Anode and Cathode?
An electrode that loses electrons is called an anode where this process is known as Oxidation.
An electrode that gains elections is called a cathode where this process is known as Reduction.
Oxidation or loss of electrons occurs at the anode, and the
The reduction or gain of electrons occurs at the cathode.
The overall reduction-oxidation reaction is called a redox reaction which happens through the application of electrical energy.
The electrolytic cell consists of three parts.
1. Electrolyte
2. Electrode (cathode)
3. Electrode (anode)
The electrolyte is usually a solution of water or other solvents in which ions are dissolved.
Salts like sodium chloride which can also be considered to be an electrolyte, when driven by an external voltage applied to the electrodes, the ions in the electrolyte are attracted to the oppositely charged electrode, where the charge transfer (also known as redox) reactions takes place.
The electrolytic cell decomposes the standard stable or inactive chemical compound in the solution when the proper polarity of electrical voltage and enough magnitude is applied.
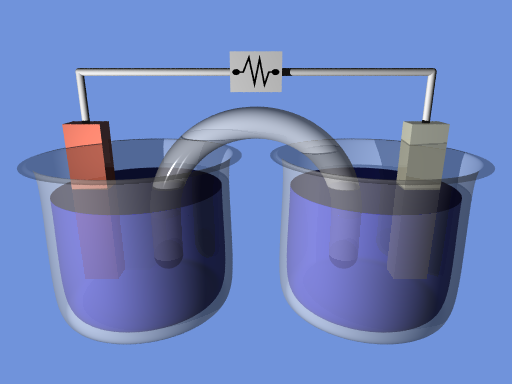
Galvanic cells (Voltaic Cells):
Galvanic cells use chemical reactions to generate electrical energy. In the galvanic cell, electrical energy is produced by a chemical redox reaction rather than being produced by electricity.
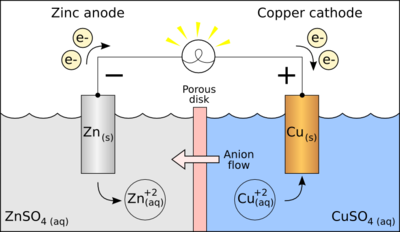
The best example of a redox reaction for a galvanic cell is the reaction between aqueous solutions of zinc (Zn) and copper (Cu):
The Galvanic Cell Equation:
Zn + Cu2+ → Zn2+ + Cu
In this cell, zinc is oxidized and copper is reduced. Initially, this causes the flow of electrons through the wire connected to two different electrode solutions.
But the flow stops just because the zinc solution is becoming positively charged while it is losing the electrons and where the copper solution becoming negatively charged from gaining the electrons.
Finally there will be no more negatively charged electrons would like to flow into negatively charged copper solution.
So, here a continuous flow of electrons is required which is nothing but electricity. So if the electrode solutions remain electrically neutral, this would solve the problem.
How to do this?
– By creating a salt bridge.
Salt bridge is nothing but a U-shaped tube that is filled with a concentrated salt solution. We can make them remain electrically neutral bypassing this salt solution, where it provides a way for ions to travel between the 2 electrode solutions. Finally this will enable the continuous flow of electrons.